Science
Magnetoelectric Nanoparticles Show Promise in Treating Pancreatic Cancer
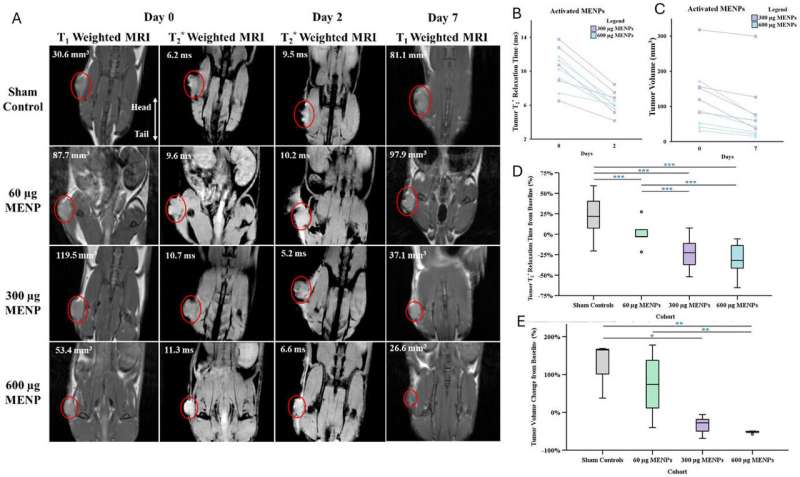
A groundbreaking study has revealed that magnetoelectric nanoparticles (MENPs) can effectively target and shrink pancreatic tumors in preclinical models, offering new hope for one of the most aggressive forms of cancer. The research, published on November 3, 2025, in the journal Advanced Science, demonstrates that these tiny, wirelessly controlled particles can locate and destroy tumors with minimal invasiveness.
Led by a collaborative team from the Sylvester Comprehensive Cancer Center, the University of Miami College of Engineering, Moffitt Cancer Center, and Cellular Nanomed, Inc., this study marks a significant advancement in cancer treatment methodologies.
The study noted that a single intravenous administration of MENPs, activated by a magnetic field generated within an MRI machine, resulted in pancreatic tumors shrinking to one-third of their original size. Remarkably, one-third of the treated models experienced complete tumor disappearance. Additionally, the survival time of the models more than doubled without harming adjacent healthy organs.
Unlike traditional treatments such as chemotherapy or surgery, this innovative approach does not rely on drugs, heat, or invasive techniques. Instead, MENPs are injected into the bloodstream and guided to the tumor site using a small magnet. When activated by the MRI’s magnetic field, these nanoparticles create localized electric fields that disrupt cancer cell membranes, initiating natural cell death while preserving surrounding healthy tissue.
Sakhrat Khizroev, Ph.D., a professor at the University of Miami and co-senior author of the study, emphasized the potential of this technology. “This study brings us one step closer to connecting to the human body wirelessly to help it heal in real time,” he stated. “We hope it opens a new era in medicine where technology can precisely target diseases that were once considered untreatable.”
The research illustrates how MENPs can be effectively delivered to pancreatic tumors and remotely activated, a significant leap forward in targeted cancer therapies. Once activated, the nanoparticles generate local electric fields that can differentiate between healthy and cancerous cells based on their molecular properties, selectively inducing apoptosis in malignant cells.
John Michael Bryant, M.D., another co-senior author and physician-scientist at Sylvester, remarked on the innovation, saying, “Magnetoelectric nanotherapy brings a new dimension to theranostic oncology by coupling imaging and controlled physical mechanisms of tumor treatment in real time.” This approach, positioned at the intersection of engineering, physics, and medicine, offers a safer and more personalized avenue for cancer care.
MRI scans conducted during the study confirmed the effectiveness of the treatment in reducing tumor size and provided clear imaging signals. This supports the potential of MENPs as a powerful therapeutic and diagnostic tool, or “theranostic” option, minimizing the side effects often associated with pharmaceutical interventions.
The concept of using MENPs to wirelessly control local electric fields was first introduced by Khizroev and Liang in 2011. Over the past decade, the idea has evolved through international research partnerships and technological advancements, culminating in this promising study.
Despite advancements in cancer treatment, pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, with a five-year survival rate below 10%. It is projected to become the second leading cause of cancer-related deaths in the United States by 2030. Traditional treatment options, including surgery, radiation, and chemotherapy, often endanger healthy tissues, while newer therapies like immunotherapy have achieved limited success.
One of the greatest challenges in treating PDAC has been the ability to control the electric fields that affect cancer cell growth. The new findings indicate that MENP therapy could provide a safer and more precise alternative for patients in the future.
“If this technology translates successfully to humans, it could change the way we think about treating pancreatic and other solid tumors,” remarked Liang, Ph.D., co-founder of Cellular Nanomed and a co-author of the study.
While the current research was conducted in preclinical models, the authors are optimistic that these findings will pave the way for future clinical trials, heralding a new generation of wireless nanomedicine in cancer treatment.
-

 Health2 months ago
Health2 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health2 months ago
Health2 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science2 weeks ago
Science2 weeks agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science2 weeks ago
Science2 weeks agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science2 weeks ago
Science2 weeks agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment4 months ago
Entertainment4 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-

 Science1 week ago
Science1 week agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 World2 months ago
World2 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 Entertainment3 months ago
Entertainment3 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 Science1 week ago
Science1 week agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment4 months ago
Entertainment4 months agoLove Island Star Toni Laite’s Mother Expresses Disappointment Over Coupling Decision
-

 Entertainment2 months ago
Entertainment2 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025









