Science
Researchers Unveil Advanced Copper Nanocluster for CO2 Reduction
In a notable advancement towards carbon neutrality, researchers from Tohoku University, Tokyo University of Science, and Vanderbilt University have developed a novel copper nanocluster that demonstrates exceptional stability and selectivity in the electrochemical conversion of carbon dioxide (CO2). The findings, published in the Journal of the American Chemical Society on June 26, 2025, reveal a breakthrough in catalysis that could significantly enhance CO2 reduction processes.
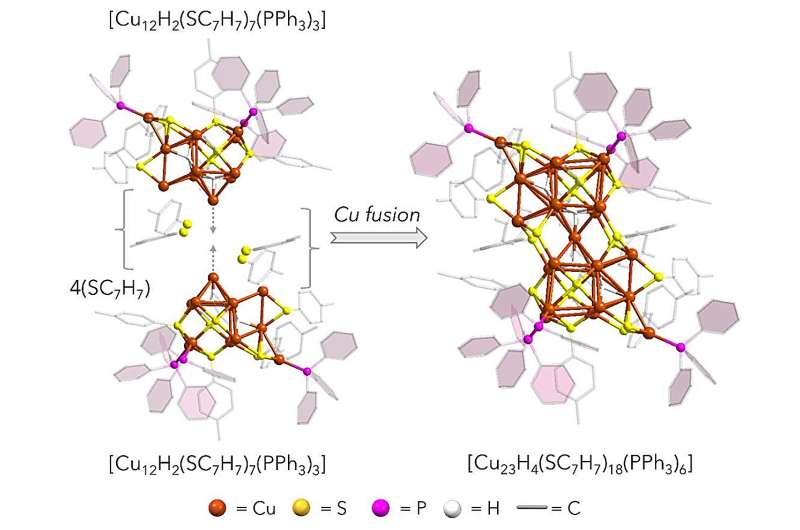
The innovative nanocluster, referred to as Cu23, combines the advantages of two different copper oxidation states: the effective but unstable Cu(0) and the stable yet less efficient Cu(I). By integrating a single Cu(0) atom into a Cu(I)-dominated framework, researchers altered the electronic structure of the material, yielding promising results. Professor Yuichi Negishi of Tohoku University emphasized the significance of this approach, stating, “This study demonstrates a new paradigm in the design of metal nanoclusters.”
The team utilized carbon black as a support material and conducted controlled-potential electrolysis under a CO2 atmosphere. At an applied potential of −1.2 V versus RHE, the Cu23 nanocluster achieved a Faradaic efficiency of approximately 26% for formic acid and 2.6% for carbon monoxide. This represents a considerable improvement in product selectivity, particularly for the more desirable formic acid compared to unwanted byproducts.
Technological Insights and Implications
Computational analyses have confirmed that the Cu23 cluster facilitates the production of a favorable intermediate at a lower limiting potential than previously recorded Cu(I)-based nanoclusters. Surprisingly, the integration of a single Cu(0) atom, though secluded within the cluster, enhances the accessibility of active sites on the nanocluster’s surface, promoting better reactant interaction.
Density Functional Theory (DFT) calculations played a pivotal role in identifying how this embedded Cu(0) atom stabilizes the crucial *HCOO intermediate necessary for formic acid production. The study also highlighted the remarkable stability of the material even after catalytic reactions, suggesting a viable path for practical applications.
This groundbreaking research opens new avenues in CO2 utilization technologies and sets a foundation for designing advanced electrocatalysts from readily available materials. By stabilizing essential intermediates and directing product selectivity, the findings offer strategic insights for addressing global sustainability challenges.
More information on this research can be found in the article titled “Atomically Precise [Cu23H4(SC7H7)18(PPh3)6] Nanocluster: Structural Integration of Johnson Solids through a Cu(0) Center and Electrocatalytic Functionality,” published in the Journal of the American Chemical Society. The DOI for this publication is 10.1021/jacs.5c05665.
As the world increasingly aims for carbon neutrality, this research not only contributes to scientific understanding but also provides a practical framework for future developments in sustainable technologies.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health3 months ago
Health3 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science2 months ago
Science2 months agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science2 months ago
Science2 months agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science1 month ago
Science1 month agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment5 months ago
Entertainment5 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-

 Science1 month ago
Science1 month agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment4 months ago
Entertainment4 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 Science1 month ago
Science1 month agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment2 months ago
Entertainment2 months agoLewis Cope Addresses Accusations of Dance Training Advantage
-

 Entertainment3 months ago
Entertainment3 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025