Health
New AI Model Revolutionizes IVF with Enhanced Accuracy
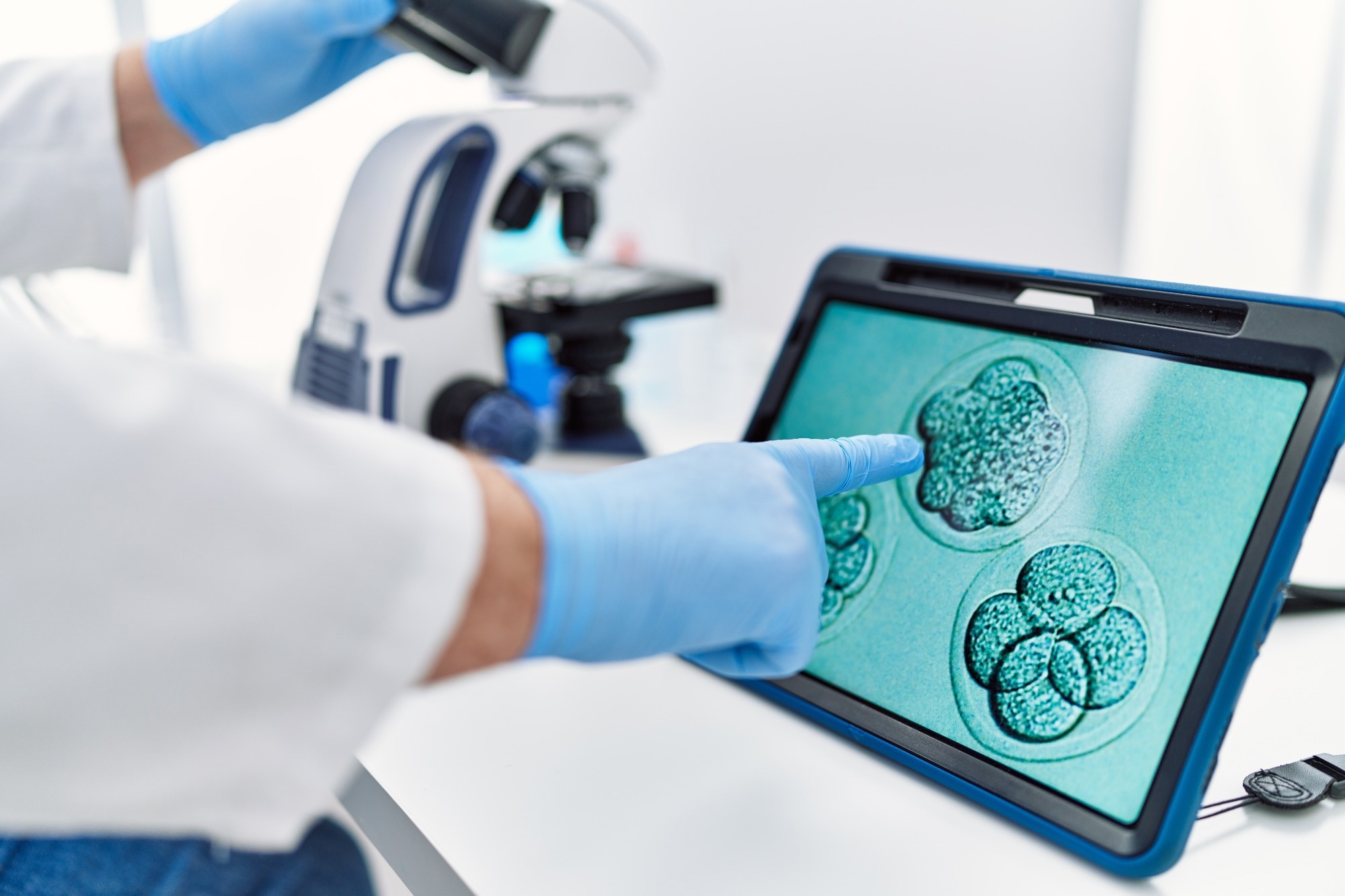
A groundbreaking study published in Nature Communications has unveiled the Foundational IVF Model for Imaging (FEMI), an innovative artificial intelligence (AI) tool designed to enhance the accuracy of embryo assessment in in vitro fertilization (IVF). By training on a dataset of approximately 18 million time-lapse images, FEMI aims to replace traditional invasive testing methods, potentially offering a faster, cheaper, and more reliable approach to selecting viable embryos.
Understanding IVF success hinges on accurate embryo assessment and selection. Traditional methods suffer from limitations, including inconsistent standards, high costs, and variable regulations regarding preimplantation genetic testing for aneuploidy (PGT-A) worldwide. These discrepancies in scoring systems and diagnostic tools can adversely influence IVF success rates, underscoring the urgent need for an efficient, non-invasive solution that alleviates the emotional and financial burdens on patients.
The Role of AI in IVF
Artificial intelligence has found a place in IVF by assisting in the prediction of embryo morphology and ploidy status, both critical for successful outcomes. Although previous deep learning models, such as STORK and ERICA, have shown promise in analyzing embryo morphology, they largely depend on image data and embryologist input. Researchers have worked to overcome these limitations by creating new models that demonstrate improved efficacy.
The Blastocyst Evaluation Learning Algorithm (BELA) can predict ploidy status without assistance from embryologists, but it is restricted to assessing embryo quality scores and ploidy status. The introduction of Vision Transformers (ViTs), which utilize a transformer-based architecture, allows for more complex pattern recognition in images and can handle large-scale datasets effectively. Nevertheless, the application of ViTs has been limited due to insufficient training dataset diversity.
The FEMI model employs the Vision Transformer masked autoencoder (ViT MAE) to facilitate self-supervised learning (SSL), reconstructing original images from masked inputs. This encoder-decoder structure promotes the understanding of essential characteristics within the dataset.
Performance and Clinical Implications
FEMI was trained on a diverse selection of time-lapse images captured from multiple clinics, specifically focusing on images taken after 85 hours post-insemination. To enhance feature learning, images were closely cropped around embryos, and the dataset was split into 80% for training and 20% for validation.
The study assessed FEMI’s predictive accuracy across various clinical tasks, including blastocyst quality scoring, ploidy prediction, blastulation time prediction, and embryo witnessing. The model’s performance was compared against several benchmark image and video-based models, including MoViNet and EfficientNet V2. Findings revealed that FEMI significantly surpassed all comparison models, particularly excelling in conditions of low embryo quality.
One notable achievement was FEMI’s accuracy in predicting ploidy status, where it performed exceptionally well, especially in predicting overall blastocyst scores and inner cell mass scores across multiple datasets. While FEMI’s success in embryo witnessing was evident, its performance in segmenting blastocyst components showed only marginal improvements compared to other models.
The ability to predict blastulation time accurately is crucial for embryologists, facilitating assessments of embryo quality and planning for subsequent procedures. In this regard, FEMI achieved a top-1 accuracy of 60.31%, closely trailing behind Embryovision’s 60.58% accuracy, highlighting the model’s competitive edge in the field.
Despite these promising results, the authors acknowledge limitations in the study. The segmentation and stage prediction tasks utilized the same datasets for training and testing, potentially affecting generalizability. Additionally, ploidy prediction tasks did not include mosaic embryos and were limited to data collected up to 112 hours post-insemination, despite viable embryos often developing beyond this timeframe. Moreover, the datasets primarily originated from high-resource clinics, which could restrict FEMI’s immediate application in lower-resource environments.
Notwithstanding these challenges, the authors advocate for FEMI’s potential as a foundational model, suggesting that it could serve as a backbone for other clinical prediction tasks—such as implantation and live birth—pending access to relevant labels. This study positions FEMI as a promising advancement in standardizing embryo assessment in IVF, with its self-supervised learning capabilities allowing it to generalize effectively across tasks.
As further validation and clinical trials are conducted, FEMI could evolve into a powerful decision-support system in reproductive medicine, ultimately improving IVF success rates and enhancing patient experiences.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health3 months ago
Health3 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science1 month ago
Science1 month agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science1 month ago
Science1 month agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science4 weeks ago
Science4 weeks agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment4 months ago
Entertainment4 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-

 Science4 weeks ago
Science4 weeks agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment4 months ago
Entertainment4 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 Science4 weeks ago
Science4 weeks agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment4 months ago
Entertainment4 months agoLove Island Star Toni Laite’s Mother Expresses Disappointment Over Coupling Decision
-

 Entertainment3 months ago
Entertainment3 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025









