Health
New Guidelines Transform Treatment Approaches for Central Sleep Apnea
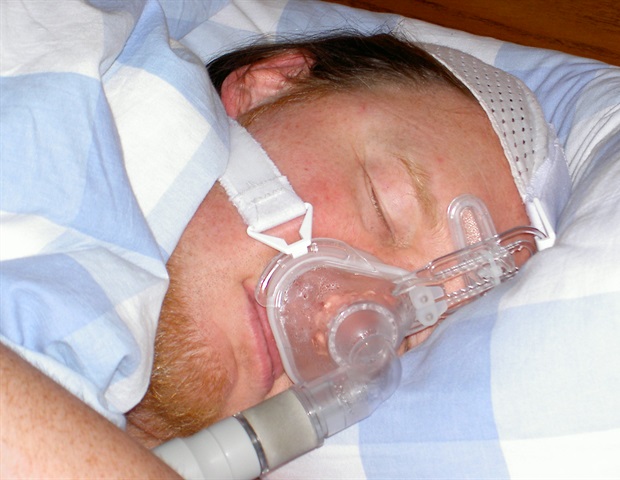
A newly released clinical practice guideline from the American Academy of Sleep Medicine (AASM) offers updated recommendations for treating central sleep apnea. This guideline, which is available online as an accepted paper in the Journal of Clinical Sleep Medicine, revises the AASM’s previous recommendations published in 2012 and 2016.
The updated guideline incorporates findings from recent research, including new insights on adaptive servo ventilation and the introduction of transvenous phrenic nerve stimulation as a novel treatment option. Central sleep apnea, characterized by the cessation or reduction of breathing during sleep, presents a complex challenge that necessitates a tailored, patient-centered approach.
Dr. M. Safwan Badr, who is the lead author of the guideline and chair of the AASM task force, emphasized the importance of focusing on enhancing quality of life and functional outcomes for patients. He noted that the goal should not solely be the elimination of disordered breathing events.
Central sleep apnea can disrupt sleep due to a lack of breathing effort and associated airflow reduction. This condition often occurs in patients with heart failure, obstructive sleep apnea, or those using opioids. The underlying causes of central sleep apnea can vary widely, which makes individualized treatment essential.
The guideline presents nine clinical recommendations, all designated as “conditional.” This classification indicates that while the recommendations are informed by current evidence, they require clinicians to apply their judgment and consider individual patient preferences. The recommendations support six treatment options tailored to specific causes of central sleep apnea:
– Continuous positive airway pressure (CPAP)
– Bilevel positive airway pressure with a backup rate
– Adaptive servo ventilation
– Low-flow oxygen
– Oral acetazolamide
– Transvenous phrenic nerve stimulation
When determining the optimal therapy for patients, clinicians are urged to consider the underlying conditions that contribute to breathing instability. Notably, adaptive servo ventilation received a conditional recommendation for patients with central sleep apnea due to multiple etiologies. However, the guideline advises that treatment in patients with heart failure and reduced ejection fraction should be conducted in specialized centers, ensuring close monitoring and follow-up, given concerns raised by a specific clinical trial.
The guideline also introduces a conditional recommendation for transvenous phrenic nerve stimulation for both primary central sleep apnea and that related to heart failure. This treatment involves an implantable device that continuously monitors and stabilizes breathing. Approved by the Food and Drug Administration in 2017 for treating moderate to severe central sleep apnea in adults, it is important to note that this invasive procedure may not be accessible to all patients and is associated with significant costs. Therefore, the guideline suggests considering less invasive treatment options first.
To develop these recommendations, the AASM assembled a task force of sleep medicine specialists with expertise in managing central sleep apnea. Their recommendations were based on a systematic review of the literature and an assessment of the evidence using the GRADE process. This approach evaluated the certainty of evidence, benefits and risks, patient values, and resource utilization. Following a public comment period, the AASM board of directors approved the final recommendations.
The guideline has received endorsements from various organizations, including the Alliance of Sleep Apnea Partners, the American Association for Respiratory Care, and the European Respiratory Society, among others.
The co-authors of the guideline include prominent figures in sleep medicine such as Dr. Rami N. Khayat, Dr. J. Shirine Allam, and Dr. Grace Pien. Notably, Dr. Randerath, one of the contributors, has disclosed that he received support for a speaking engagement from Philips Respironics but did not participate in the voting for the adaptive servo ventilation recommendation. Other task force members reported no relevant conflicts of interest.
For further information, copies of the clinical practice guideline titled “Treatment of Central Sleep Apnea in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline,” as well as the systematic review and GRADE assessment, can be requested via email at [email protected]. The guideline was posted as an accepted paper on August 18, 2023, and is set to appear in the December 2023 issue of the Journal of Clinical Sleep Medicine.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health3 months ago
Health3 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science1 month ago
Science1 month agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science1 month ago
Science1 month agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science4 weeks ago
Science4 weeks agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment4 months ago
Entertainment4 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-

 Science4 weeks ago
Science4 weeks agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment4 months ago
Entertainment4 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 Science4 weeks ago
Science4 weeks agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment4 months ago
Entertainment4 months agoLove Island Star Toni Laite’s Mother Expresses Disappointment Over Coupling Decision
-

 Entertainment3 months ago
Entertainment3 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025









