Health
Parents Urged to Stop Using Kids’ Magnesium Gummies Over Melatonin Risk
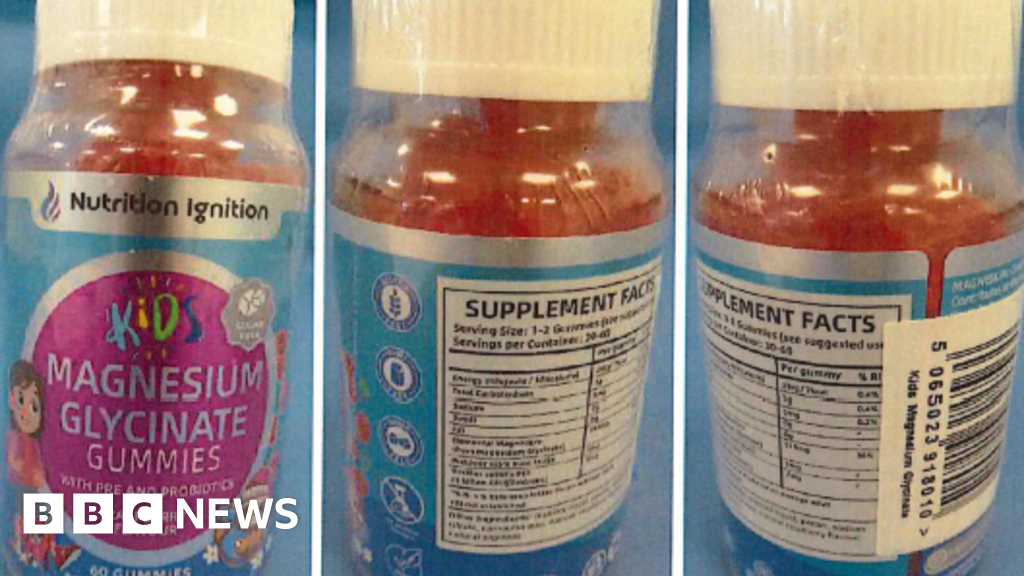
Parents have been advised to stop using Nutrition Ignition Kids Magnesium Glycinate Gummies after testing revealed the presence of an undeclared drug, melatonin. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) found that the gummies contained between 1.5 mg and 1.7 mg of melatonin, a substance that is available only by prescription and can lead to side effects such as drowsiness, headaches, dizziness, and nausea.
Following the discovery, the MHRA has removed the product from sale. Parents who have administered the gummies to their children are encouraged to consult a healthcare professional if they observe any adverse reactions. Fortunately, lasting harm is not anticipated from the consumption of these gummies.
Concerns About Melatonin in Children’s Supplements
Melatonin is often prescribed to children over the age of six for sleep management when other methods have proven ineffective. It is frequently used for conditions like attention deficit hyperactivity disorder (ADHD), delayed sleep-wake phase disorder (DSWPD), and for short-term treatment of insomnia. Typical starting doses for children range from 1 mg to 5 mg per day, and research has not identified serious side effects in pediatric studies.
The gummies in question were marketed for children aged four and above, promoting benefits such as enhanced calmness, focus, and digestion. Magnesium glycinate, the primary ingredient, is reputed to support muscle function, improve sleep quality, and regulate the nervous system.
Parents are advised to dispose of the gummies at a local pharmacy and report any side effects through the MHRA’s Yellow Card scheme. The Nutrition Ignition brand website has been taken down, and the product has been removed from major online retailers, including Amazon and eBay.
Background and Response from Nutrition Ignition
The situation came to light when two concerned mothers noticed their children were falling asleep more quickly than usual after consuming the gummies. This prompted them to have the product tested, leading to the revelation of melatonin’s presence. Sally Westcott, the brand’s owner and an NHS clinical therapy lead based in Surrey, was ordered by the MHRA to remove the raspberry-flavored gummies from the market over a month ago. In a previous statement made in June, Westcott asserted that she had “never knowingly sold products containing undeclared ingredients.”
As the investigation unfolds, parents are left with a critical reminder to carefully scrutinize the ingredients of supplements marketed for children. The safety and well-being of young consumers must always remain a priority.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health3 months ago
Health3 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science1 month ago
Science1 month agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science1 month ago
Science1 month agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science4 weeks ago
Science4 weeks agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment4 months ago
Entertainment4 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-

 Science4 weeks ago
Science4 weeks agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment4 months ago
Entertainment4 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 Science4 weeks ago
Science4 weeks agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment4 months ago
Entertainment4 months agoLove Island Star Toni Laite’s Mother Expresses Disappointment Over Coupling Decision
-

 Entertainment3 months ago
Entertainment3 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025









