Business
Lonza Unveils New TheraPEAK® Products to Enhance Gene Therapy
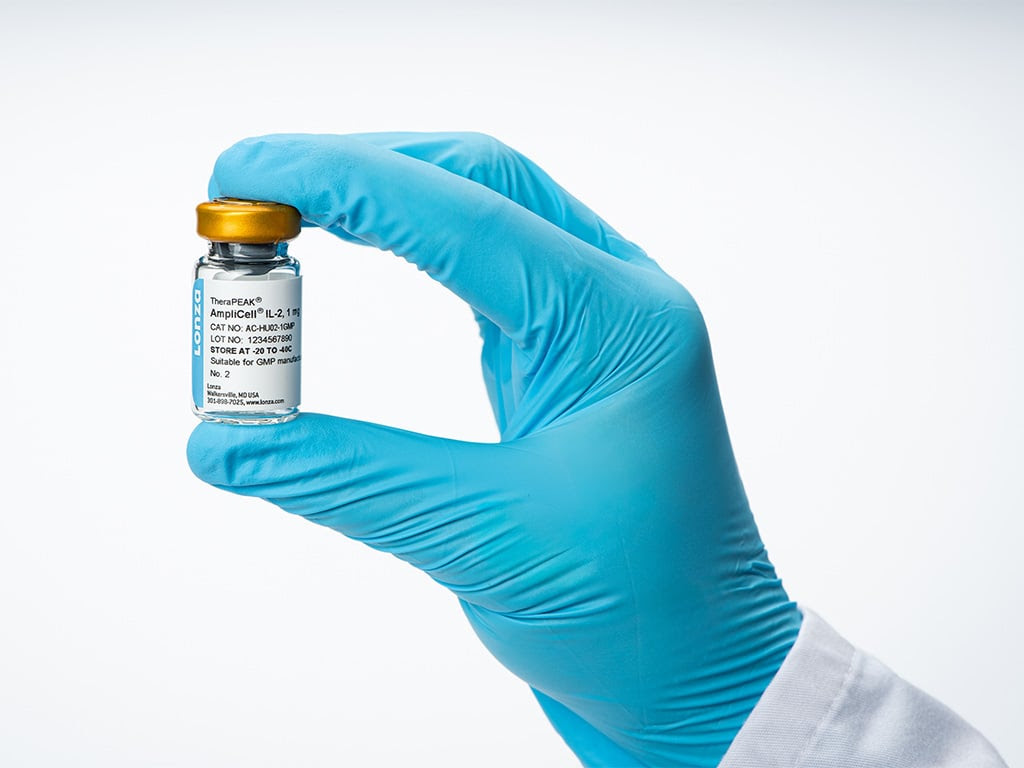
Lonza has announced the launch of two new products under its TheraPEAK® brand, aimed at advancing the manufacturing processes for cell and gene therapy. The new offerings, TheraPEAK® AmpliCell® Cytokines and TheraPEAK® 293-GT® Medium, are designed to enhance immune cell expansion, activation, and the production of adeno-associated viruses (AAV) in both research and Good Manufacturing Practice (GMP) settings.
The AmpliCell® Cytokines are derived from a mammalian expression system, ensuring a high level of biological activity. These cytokines support the expansion, activation, and differentiation of immune cells while maintaining proper folding and glycosylation. This results in a native-like structure and function that is unattainable through bacterial systems. The product is engineered to provide consistent batch-to-batch performance, which is vital for sensitive applications.
In conjunction with this, the TheraPEAK® 293-GT® Medium is a chemically defined, animal-origin-free medium specifically optimized for AAV production in suspension HEK293 cells. This medium integrates seamlessly with existing workflows and supports commercially available transfection reagents. It enables robust AAV titers while achieving high full-to-empty capsid ratios, making it a scalable and convenient solution for gene therapy programs.
Impact on Cell and Gene Therapy Development
The introduction of these new products marks a significant expansion of Lonza’s capabilities in the cell and gene therapy sector. According to the company, the TheraPEAK® products have already been utilized in FDA-approved therapies and over 130 clinical trials globally. These innovations are expected to streamline workflows from initial discovery phases through to clinical development.
Mike Goetter, Head of Bioscience, Specialized Modalities at Lonza, emphasized the importance of these products: “The introduction of AmpliCell® Cytokines and the 293-GT® Medium to our TheraPEAK® Range greatly enhances our offering for cell and gene therapy customers, providing them with reliable solutions to support their drug development efforts.”
The launch signifies Lonza’s commitment to supporting the evolving needs of the biopharmaceutical industry, particularly in the increasingly important fields of cell and gene therapies. By providing high-performance and GMP-compatible tools, Lonza aims to facilitate advancements in therapeutic development, ultimately benefiting patients worldwide.
As the demand for effective and reliable therapies continues to rise, these new additions to the TheraPEAK® portfolio could play a critical role in enhancing productivity and efficiency in the manufacturing of cutting-edge treatments.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health3 months ago
Health3 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science1 month ago
Science1 month agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science1 month ago
Science1 month agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science4 weeks ago
Science4 weeks agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment4 months ago
Entertainment4 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-
 Science4 weeks ago
Science4 weeks agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment4 months ago
Entertainment4 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-
 Science4 weeks ago
Science4 weeks agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor
-

 Entertainment4 months ago
Entertainment4 months agoLove Island Star Toni Laite’s Mother Expresses Disappointment Over Coupling Decision
-

 Entertainment3 months ago
Entertainment3 months agoMajor Cast Changes at Coronation Street: Exits and Returns in 2025









