Top Stories
Sarepta Suspends Elevidys Shipments Following FDA Intervention

Sarepta Therapeutics has agreed to halt shipments of its gene therapy product, Elevidys (delandistrogene moxeparvovec), in response to a request from the United States Food and Drug Administration (FDA). This decision, made on July 22, 2023, follows earlier communications from the FDA on July 17, where the agency expressed concerns regarding patient safety.
Initially, Sarepta rejected the FDA’s request, maintaining that no new safety signals had been identified. However, CEO Doug Ingram later emphasized the importance of maintaining a “positive working relationship” with the regulatory agency. He described the decision to pause shipments as “painful,” particularly given that individuals suffering from Duchenne muscular dystrophy (DMD) are in urgent need of effective treatment options.
“We made this decision to ensure that we can address any questions the FDA may have and to complete the Elevidys label supplement process,” Ingram stated. The company confirmed that it will collaborate with the FDA to respond to information requests while progressing the safety labeling supplement for Elevidys.
Patient Safety Concerns Prompt FDA Action
The FDA’s intervention comes in light of serious safety concerns. On July 17, a third patient death connected to Sarepta’s gene therapy was reported. A 51-year-old male with limb-girdle muscular dystrophy type 2D/R3 passed away after treatment, marking a troubling turn for the biopharmaceutical firm. This case was disclosed by media outlets and subsequently announced to investors the following day.
The FDA’s communication on July 17 also included a clinical hold on studies for another treatment under development, known as SRP-9004. This decision follows two previously reported patient deaths related to Elevidys, both attributed to acute liver failure (ALF). The initial death was reported in March 2025, followed by another in June 2025.
Both Elevidys and SRP-9004 utilize the same recombinant adeno-associated viral vector, AAVrh74, which raises additional questions regarding the safety profile of these therapies.
Ongoing Regulatory Engagement
In light of these developments, Sarepta is actively engaging with the FDA to update the label for Elevidys. This includes the addition of a black box warning for acute liver injury (ALI) and ALF, indicating a heightened awareness of the potential risks associated with the treatment.
Despite the challenges, Ingram remains focused on the company’s commitment to patient needs and regulatory compliance. “It is our duty to ensure that we are transparent and diligent in our communications with the FDA,” he remarked.
As the situation evolves, Sarepta is expected to provide updates on its progress and any changes to the status of Elevidys and related therapies. The company’s ability to navigate this regulatory landscape will be crucial for its future and for the patients who rely on its innovative treatments.
-

 Health3 months ago
Health3 months agoNeurologist Warns Excessive Use of Supplements Can Harm Brain
-

 Health4 months ago
Health4 months agoFiona Phillips’ Husband Shares Heartfelt Update on Her Alzheimer’s Journey
-

 Science2 months ago
Science2 months agoBrian Cox Addresses Claims of Alien Probe in 3I/ATLAS Discovery
-

 Science2 months ago
Science2 months agoNASA Investigates Unusual Comet 3I/ATLAS; New Findings Emerge
-

 Science2 months ago
Science2 months agoScientists Examine 3I/ATLAS: Alien Artifact or Cosmic Oddity?
-

 Entertainment2 months ago
Entertainment2 months agoLewis Cope Addresses Accusations of Dance Training Advantage
-

 Entertainment5 months ago
Entertainment5 months agoKerry Katona Discusses Future Baby Plans and Brian McFadden’s Wedding
-
 Science2 months ago
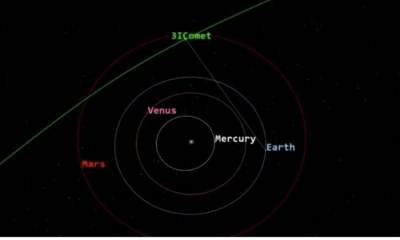
Science2 months agoNASA Investigates Speedy Object 3I/ATLAS, Sparking Speculation
-

 Entertainment5 months ago
Entertainment5 months agoEmmerdale Faces Tension as Dylan and April’s Lives Hang in the Balance
-

 World3 months ago
World3 months agoCole Palmer’s Cryptic Message to Kobbie Mainoo Following Loan Talks
-

 World4 weeks ago
World4 weeks agoBailey and Rebecca Announce Heartbreaking Split After MAFS Reunion
-
 Science2 months ago
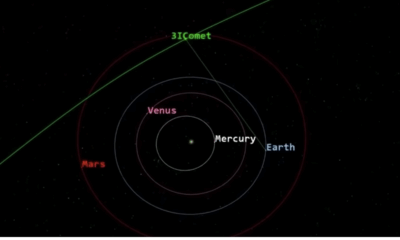
Science2 months agoNASA Scientists Explore Origins of 3I/ATLAS, a Fast-Moving Visitor